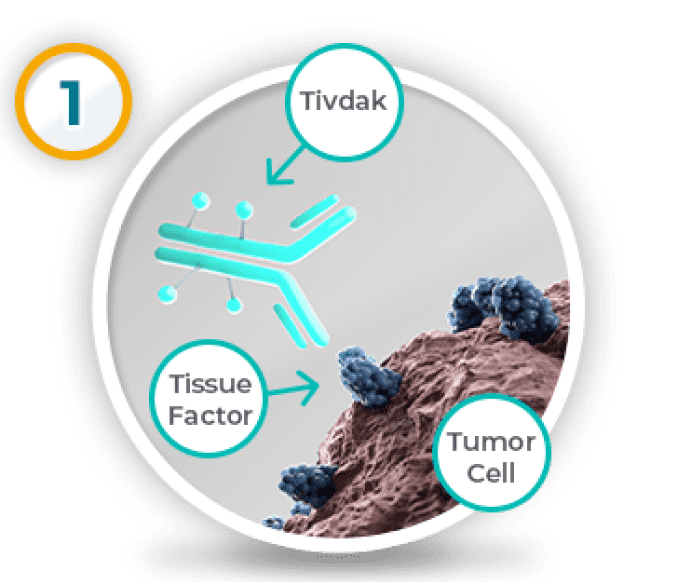
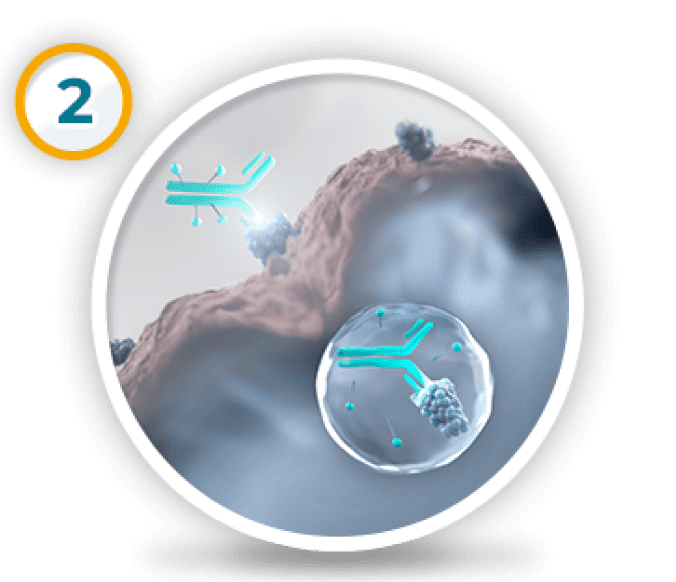
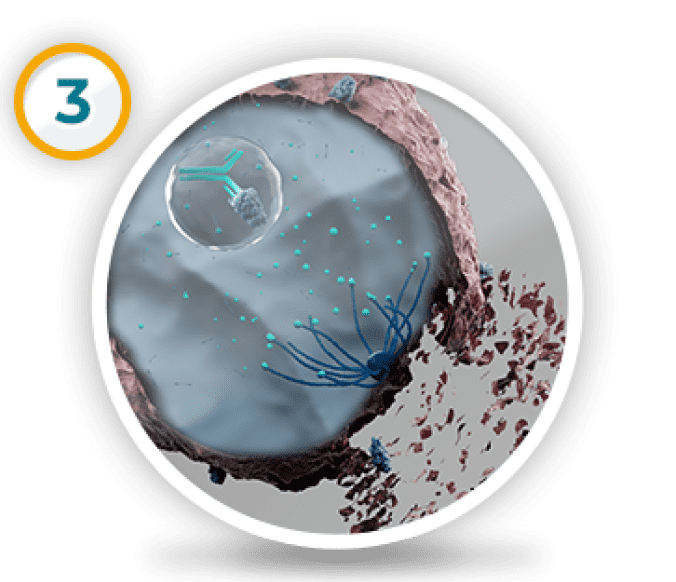
ALISHA:
I’m an artist. I’m a hairstylist and I own my own salon. I get a lot of happiness
when
someone feels good in their new haircut.
Cutting hair and helping others has always felt like my purpose. It’s also important
for
me to find inspiration in life.
I use my painting to escape and to be inspired. I’m in the moment, focused on what
I’m
creating.
Drip painting is a way of painting that you pour paint straight onto the canvas.
There’s
just so many different ways to create something. No two paintings ever turn out
exactly
the same.
I started painting to process everything going on in my life when I was diagnosed
with
cancer.
When I was 30, my doctor found a tumor pressing up against my reproductive organs. I
underwent some tests, including a Pap smear, and it showed I had cervical cancer.
I received my initial treatment and, afterwards, my doctor said there was no more
signs
of cancer. For 22 years, I received regular scans and testing.
One day I experienced unexplained bleeding, and I knew I needed to see my doctor. A
biopsy showed that my cervical cancer was back.
After that diagnosis, I was devastated. I was scared. I was scared of dying, I just
really felt alone.
After many rounds of chemotherapy, the treatment stopped working and I wasn’t sure
what
to do next.
I started to get discouraged. It was so scary and so hard. I just needed something,
an
outlet, to leave it. I turned to my art to process the emotions I was feeling.
It’s just so beautiful to create something and then, just, you don’t think about it.
You
don’t think about anything, no problems, no nothing. It’s just creating.
The art is taking over. I let my emotions spill out onto the canvas. Immersing myself
into my art allowed me to stay positive during this time.
I knew I wasn’t ready to give up. I knew it was going to be a fight, but I made a
choice
to keep trying. That’s when my doctor recommended Tivdak.
Narrator:
TIVDAK (tisotumab vedotin-tftv) 40mg, for injection is a prescription medicine used
to
treat adults with cervical cancer that has returned or has spread to other parts of
the
body, and who have received chemotherapy that did not work or is no longer working.
Alisha:
My first reaction when I heard about TIVDAK was I had to do it.
My doctor told me about the serious side effects of TIVDAK. I was concerned about the
side effects, but I was also concerned about losing my fight with cancer. Giving up
was
not an option.
After talking with my doctor, I learned about the steps that can help reduce the risk
of
eye problems. I felt more confident in my knowledge, and I decided to start
treatment on
TIVDAK.
Narrator:
Eye problems are common with TIVDAK and can be severe. TIVDAK can cause changes to
the
surface of your eye that can lead to dry eyes, eye redness, eye irritation, corneal
ulcers, blurred vision, and severe vision loss.
Serious side effects of TIVDAK may include eye problems, nerve problems (peripheral
neuropathy), bleeding problems (hemorrhage), lung problems, and severe skin
reactions.
TIVDAK can harm your unborn baby.
These are not all the side effects of TIVDAK. Please see the end of this video for
more
details about side effects and visit TIVDAK.com/resources to review the Important
Facts
about TIVDAK including the IMPORTANT WARNING.
Alisha:
I visit an eye specialist before every infusion. After setting up a routine with my
eye
specialist, I feel good about the process.
My treatment takes about 30 minutes. During that time, I like to sit back and listen
to
music.
I had my first scan a few months after starting treatment. It showed that my cervical
cancer was responding to treatment.
I continue to go in for my infusions every three weeks. I experience some side
effects,
like nausea, dry, irritated eyes, but my doctor helped me manage them.
There are additional side effects that you might experience on treatment. This is
just my
experience, everyone’s experience on TIVDAK is different.
I’m so happy that I can continue finding my purpose by helping others.
When cancer patients come into my salon, I love helping them feel confident with how
they
look.
I now approach my art with a new energy. I’m able to keep trying new things for
inspiration.
I love discovering all the things I’m able to do through my art.
VOICEOVER:
What is TIVDAK?
TIVDAK is a prescription medicine used to treat adults with cervical cancer:
- that has returned or has spread to other parts of the body, and
- who have received chemotherapy that did not work or is no longer working.
Important Safety Information
What is the most important information I should know about
TIVDAK?
Eye problems are common with TIVDAK and can be severe.
TIVDAK
can cause changes to the surface of your eye that can lead to dry eyes, eye redness,
eye
irritation, corneal ulcers, blurred vision, and severe vision loss. Tell your healthcare provider if you develop new or worsening
vision
changes or eye problems during treatment.
Your healthcare provider will send you to an eye specialist to check your eyes before
you
start treatment with TIVDAK, before each infusion for your first 9 infusions of
TIVDAK,
and as needed for any new or worsening signs or symptoms of eye problems. Your
healthcare provider will ask if you have any signs or symptoms of eye problems
before
each infusion. You will be referred to an eye specialist for any new or worsening
signs
or symptoms of eye problems.
Your healthcare provider will prescribe 3 different types of eye drops before you
start
treatment with TIVDAK. Bring the eye drops with you to each
infusion
and use them as directed by your healthcare provider to reduce your risk of eye
problems:
- Use 1 drop of steroid eye drops in each eye before each infusion and continue to
use
your eye drops 3 times a day for 3 days after each infusion
- Use vasoconstrictor eye drops right before each infusion
- Use lubricating eye drops throughout treatment and for 30 days after your last
dose
of TIVDAK
Do not wear contact lenses throughout your treatment with
TIVDAK unless you are told to use them by your eye specialist.
What are the possible side effects of TIVDAK?
Serious side effects of TIVDAK may include:
Eye problems. See “What is the most
important information I should know about TIVDAK?”
Nerve problems (peripheral neuropathy) are common with
TIVDAK
and can be serious. Tell your healthcare provider right away if you have new or
worsening numbness or tingling in your hands or feet or muscle weakness.
Bleeding problems (hemorrhage) are common with TIVDAK and
can
be serious. Tell your healthcare provider or get medical help right away if you have
signs or symptoms of bleeding during treatment with TIVDAK, including blood in your
stools or black stools (looks like tar), blood in your urine, cough up or vomit
blood,
unusual vaginal bleeding, or any unusual or heavy bleeding.
Lung problems. TIVDAK may cause severe or life-threatening
inflammation of the lungs that can lead to death. Tell your healthcare provider
right
away if you have new or worsening symptoms, including trouble breathing, shortness
of
breath, or cough.
Severe skin reactions. TIVDAK may cause severe or
life-threatening skin reactions that can lead to death. Tell your healthcare
provider or
get medical help right away if you have signs or symptoms of a severe skin reaction
during treatment with TIVDAK, including:
- Skin reactions that look like rings (target lesions)
- Rash or itching that continues to get worse
- Blistering or peeling of the skin
- Painful sores or ulcers in your mouth, nose, throat, or genital area
- Fever or flu-like symptoms
- Swollen lymph nodes
The most common side effects of TIVDAK include:
- Decreased red blood cell counts
- Numbness or tingling in your hands or feet
- Eye problems (conjunctival disorders)
- Nausea
- Tiredness
- Changes in liver function blood tests
- Nosebleed
- Hair loss (alopecia)
- Bleeding (hemorrhage)
Your healthcare provider may decrease your dose of TIVDAK, temporarily stop, or
completely stop treatment if you have side effects.
What should I tell my healthcare provider before receiving
TIVDAK?
Tell your healthcare provider about all your medical conditions,
including if you:
- have a history of vision or eye problems
- have numbness or tingling in your hands or feet
- have bleeding problems
- have liver problems
- are pregnant or plan to become pregnant; TIVDAK can harm your unborn baby. Tell
your
healthcare provider right away if you become pregnant or think you may be
pregnant
during treatment with TIVDAK
- are breastfeeding (nursing) or plan to breastfeed. It is not known if TIVDAK
passes
into your breast milk. Do not breastfeed during treatment and for at least 3
weeks
after the last dose of TIVDAK
Females who can become pregnant:
- Your healthcare provider should do a pregnancy test before you start treatment
with
TIVDAK
- Use an effective method of birth control during your treatment and for at least
2
months after the last dose of TIVDAK
Males with female partners who can become pregnant:
- Use effective birth control during treatment and for 4 months after the last
dose of
TIVDAK
Tell your healthcare provider about all the medicines you
take,
including prescription and over-the-counter medicines, vitamins, and herbal
supplements.
Taking TIVDAK with certain other medicines may cause side effects.
These are not all of the possible side effects of TIVDAK. Discuss side effects with
your
healthcare provider. You may report side effects to FDA at 1-800-FDA-1088.
Please see the full Prescribing Information with Medication Guide about TIVDAK
including
an IMPORTANT WARNING at TIVDAK.com.