
Is Tivdak right for you?
When cancer spreads or comes back, treatments you have taken before for your cervical cancer may not be right for you anymore. Tivdak is for adults who have cervical cancer that has returned or has spread and have had chemotherapy that did not work or is no longer working.
Diagnosis may come late
In 16% of patients, cervical cancer has already spread to other parts of the body at diagnosis.
In up to 61% of people diagnosed with earlier stages of cervical cancer, the cancer will spread to other parts of the body within 2 years after initial treatment.
REMEMBER...YOU ARE NOT ALONE
What is Tivdak?
*Advanced cervical cancer is cancer that has returned or spread to other parts of the body while on or after anti-cancer therapy.
How Tivdak is thought to work
Based on lab studies
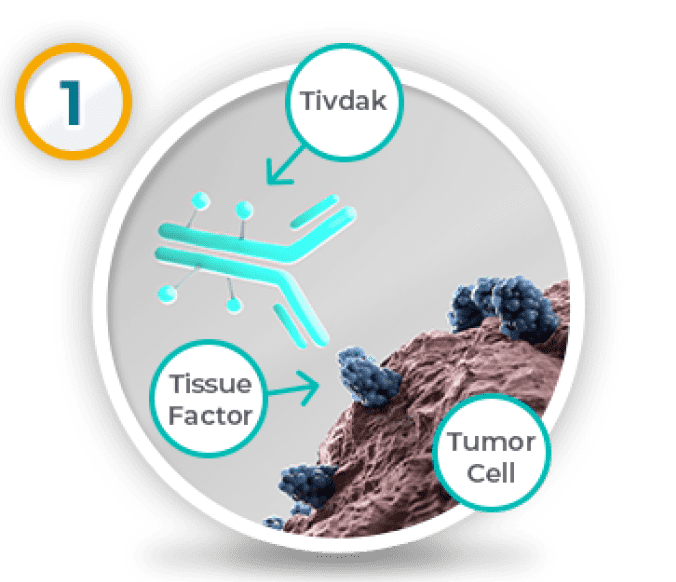
Tivdak attaches to a special protein, called Tissue Factor, on the outside of some types of tumor cells
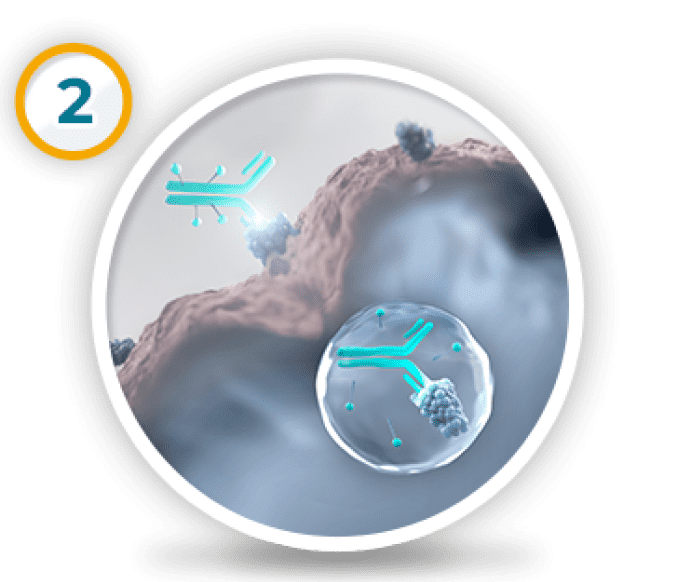
Tivdak enters the cell
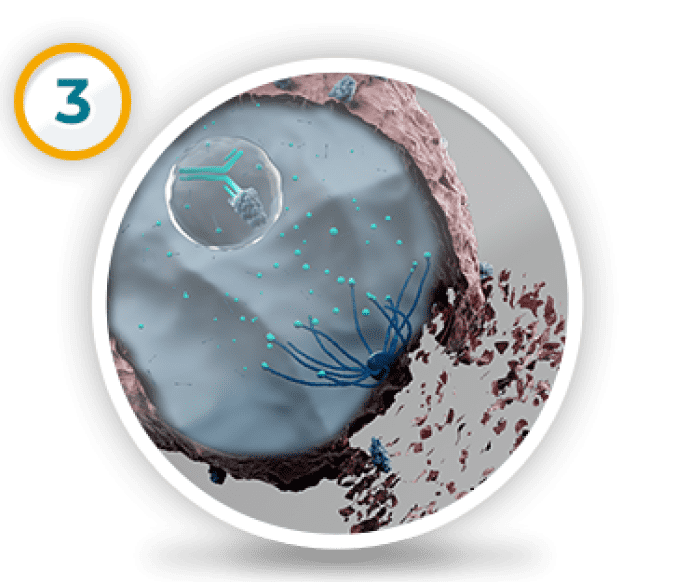
The medicine needed to kill the cell from the inside is released
What could TIVDAK DO FOR YOU?

What to expect DURING TREATMENT

Hear a real Tivdak PATIENT’S STORY

Important Safety Information
What is the most important information I should know about TIVDAK?
Eye problems are common with TIVDAK and can be severe. TIVDAK can cause changes to the surface of your eye that can lead to dry eyes, eye redness, eye irritation, corneal ulcers, blurred vision, and severe vision loss. Tell your healthcare provider if you develop new or worsening vision changes or eye problems during treatment.
Your healthcare provider will send you to an eye specialist to check your eyes before you start treatment with TIVDAK, before each infusion for your first 9 infusions of TIVDAK, and as needed for any new or worsening signs or symptoms of eye problems. Your healthcare provider will ask if you have any signs or symptoms of eye problems before each infusion. You will be referred to an eye specialist for any new or worsening signs or symptoms of eye problems.
Your healthcare provider will prescribe 3 different types of eye drops before you start treatment with TIVDAK. Bring the eye drops with you to each infusion and use them as directed by your healthcare provider to reduce your risk of eye problems:
- Use 1 drop of steroid eye drops in each eye before each infusion and continue to use your eye drops 3 times a day for 3 days after each infusion
- Use vasoconstrictor eye drops right before each infusion
- Use lubricating eye drops throughout treatment and for 30 days after your last dose of TIVDAK
Do not wear contact lenses throughout your treatment with TIVDAK unless you are told to use them by your eye specialist.
What are the possible side effects of TIVDAK?
Serious side effects of TIVDAK may include:
Eye problems. See “What is the most important information I should know about TIVDAK?”
Nerve problems (peripheral neuropathy) are common with TIVDAK and can be serious. Tell your healthcare provider right away if you have new or worsening numbness or tingling in your hands or feet or muscle weakness.
Bleeding problems (hemorrhage) are common with TIVDAK and can be serious. Tell your healthcare provider or get medical help right away if you have signs or symptoms of bleeding during treatment with TIVDAK, including blood in your stools or black stools (looks like tar), blood in your urine, cough up or vomit blood, unusual vaginal bleeding, or any unusual or heavy bleeding.
Lung problems. TIVDAK may cause severe or life-threatening inflammation of the lungs that can lead to death. Tell your healthcare provider right away if you have new or worsening symptoms, including trouble breathing, shortness of breath, or cough.
Severe skin reactions. TIVDAK may cause severe or life-threatening skin reactions that can lead to death. Tell your healthcare provider or get medical help right away if you have signs or symptoms of a severe skin reaction during treatment with TIVDAK, including:
- Skin reactions that look like rings (target lesions)
- Rash or itching that continues to get worse
- Blistering or peeling of the skin
- Painful sores or ulcers in your mouth, nose, throat, or genital area
- Fever or flu-like symptoms
- Swollen lymph nodes
The most common side effects of TIVDAK include:
- Decreased red blood cell counts
- Numbness or tingling in your hands or feet
- Eye problems (conjunctival disorders)
- Nausea
- Tiredness
- Changes in liver function blood tests
- Nosebleed
- Hair loss (alopecia)
- Bleeding (hemorrhage)
Your healthcare provider may decrease your dose of TIVDAK, temporarily stop, or completely stop treatment if you have side effects.
What should I tell my healthcare provider before receiving TIVDAK?
Tell your healthcare provider about all your medical conditions, including if you:
- have a history of vision or eye problems
- have numbness or tingling in your hands or feet
- have bleeding problems
- have liver problems
- are pregnant or plan to become pregnant; TIVDAK can harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with TIVDAK
are breastfeeding (nursing) or plan to breastfeed. It is not known if TIVDAK passes into your breast milk. Do not breastfeed during treatment and for at least 3 weeks after the last dose of TIVDAK
Females who can become pregnant:
- Your healthcare provider should do a pregnancy test before you start treatment with TIVDAK
- Use an effective method of birth control during your treatment and for at least 2 months after the last dose of TIVDAK
Males with female partners who can become pregnant:
- Use effective birth control during treatment and for 4 months after the last dose of TIVDAK
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking TIVDAK with certain other medicines may cause side effects.
These are not all of the possible side effects of TIVDAK. Discuss side effects with your healthcare provider. You may report side effects to FDA at 1-800-FDA-1088.
Please see the full Prescribing Information with Medication Guide about TIVDAK including an IMPORTANT WARNING.
What is TIVDAK?
TIVDAK is a prescription medicine used to treat adults with cervical cancer:
- that has returned or has spread to other parts of the body, and
- who have received chemotherapy that did not work or is no longer working.
Important Safety Information
What is the most important information I should know about TIVDAK?
Eye problems are common with TIVDAK and can be severe. TIVDAK can cause changes to the surface of your eye that can lead to dry eyes, eye redness, eye irritation, corneal ulcers, blurred vision, and severe vision loss. Tell your healthcare provider if you develop new or worsening vision changes or eye problems during treatment.
Your healthcare provider will send you to an eye specialist to check your eyes before you start treatment with TIVDAK, before each infusion for your first 9 infusions of TIVDAK, and as needed for any new or worsening signs or symptoms of eye problems. Your healthcare provider will ask if you have any signs or symptoms of eye problems before each infusion. You will be referred to an eye specialist for any new or worsening signs or symptoms of eye problems.
Your healthcare provider will prescribe 3 different types of eye drops before you start treatment with TIVDAK. Bring the eye drops with you to each infusion and use them as directed by your healthcare provider to reduce your risk of eye problems:
- Use 1 drop of steroid eye drops in each eye before each infusion and continue to use your eye drops 3 times a day for 3 days after each infusion
- Use vasoconstrictor eye drops right before each infusion
- Use lubricating eye drops throughout treatment and for 30 days after your last dose of TIVDAK
Do not wear contact lenses throughout your treatment with TIVDAK unless you are told to use them by your eye specialist.
What are the possible side effects of TIVDAK?
Serious side effects of TIVDAK may include:
Eye problems. See “What is the most important information I should know about TIVDAK?”
Nerve problems (peripheral neuropathy) are common with TIVDAK and can be serious. Tell your healthcare provider right away if you have new or worsening numbness or tingling in your hands or feet or muscle weakness.
Bleeding problems (hemorrhage) are common with TIVDAK and can be serious. Tell your healthcare provider or get medical help right away if you have signs or symptoms of bleeding during treatment with TIVDAK, including blood in your stools or black stools (looks like tar), blood in your urine, cough up or vomit blood, unusual vaginal bleeding, or any unusual or heavy bleeding.
Lung problems. TIVDAK may cause severe or life-threatening inflammation of the lungs that can lead to death. Tell your healthcare provider right away if you have new or worsening symptoms, including trouble breathing, shortness of breath, or cough.
Severe skin reactions. TIVDAK may cause severe or life-threatening skin reactions that can lead to death. Tell your healthcare provider or get medical help right away if you have signs or symptoms of a severe skin reaction during treatment with TIVDAK, including:
- Skin reactions that look like rings (target lesions)
- Rash or itching that continues to get worse
- Blistering or peeling of the skin
- Painful sores or ulcers in your mouth, nose, throat, or genital area
- Fever or flu-like symptoms
- Swollen lymph nodes
The most common side effects of TIVDAK include:
- Decreased red blood cell counts
- Numbness or tingling in your hands or feet
- Eye problems (conjunctival disorders)
- Nausea
- Tiredness
- Changes in liver function blood tests
- Nosebleed
- Hair loss (alopecia)
- Bleeding (hemorrhage)
Your healthcare provider may decrease your dose of TIVDAK, temporarily stop, or completely stop treatment if you have side effects.
What should I tell my healthcare provider before receiving TIVDAK?
Tell your healthcare provider about all your medical conditions, including if you:
- have a history of vision or eye problems
- have numbness or tingling in your hands or feet
- have bleeding problems
- have liver problems
- are pregnant or plan to become pregnant; TIVDAK can harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with TIVDAK
are breastfeeding (nursing) or plan to breastfeed. It is not known if TIVDAK passes into your breast milk. Do not breastfeed during treatment and for at least 3 weeks after the last dose of TIVDAK
Females who can become pregnant:
- Your healthcare provider should do a pregnancy test before you start treatment with TIVDAK
- Use an effective method of birth control during your treatment and for at least 2 months after the last dose of TIVDAK
Males with female partners who can become pregnant:
- Use effective birth control during treatment and for 4 months after the last dose of TIVDAK
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking TIVDAK with certain other medicines may cause side effects.
These are not all of the possible side effects of TIVDAK. Discuss side effects with your healthcare provider. You may report side effects to FDA at 1-800-FDA-1088.
Please see the full Prescribing Information with Medication Guide about TIVDAK including an IMPORTANT WARNING.
What is TIVDAK?
TIVDAK is a prescription medicine used to treat adults with cervical cancer:
- that has returned or has spread to other parts of the body, and
- who have received chemotherapy that did not work or is no longer working.
You are now leaving
tivdak.com
You are now leaving tivdak.com
By clicking this link, you will be redirected to a website that is neither owned nor controlled by Pfizer or Genmab. Pfizer and Genmab are not responsible for the content or services of this site.
No resource selected
Please select 1 or more resources.
